
Supporting Florida’s Innovation Economy

Amend Surgical Receives Funding for Suture-less Nerve Repair Device
Alachua-based Company Accelerates Development and Market Launch of Tissure™
Alachua, FL - April 11, 2023 /PRNewswire/ -- Amend Surgical, Inc., an Alachua-based medical device company, announced today that it has been awarded a substantial product development funding from The Michigan-Pittsburgh-Wyss Regenerative Medicine (MPWRM) Resource Center to commercialize a novel, suture-less solution for the repair of peripheral nerves. Tissure is a resorbable hydrogel membrane with a chitosan-based adhesive that has the adhesion and mechanical strength to potentially replace suture for the repair of peripheral nerve injuries. Tissure is easy to apply and will greatly simplify this challenging surgical procedure. Amend Surgical exclusively licensed the rights for this technology from the Wyss Institute at Harvard University in 2020.
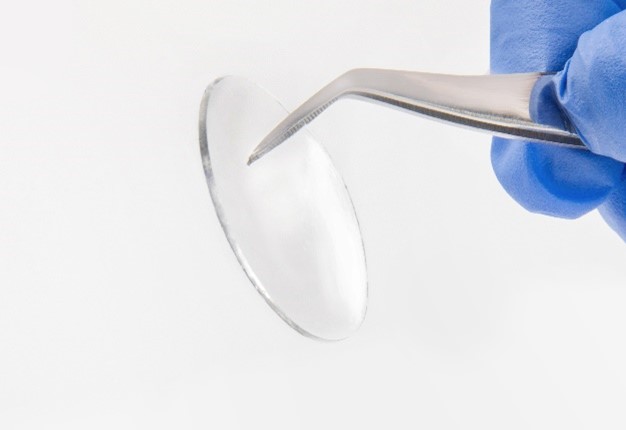
The use of suture to repair peripheral nerve injuries is the current standard of care. However, any device that penetrates the surface of a nerve can traumatize and lead to scar tissue that may impede nerve regeneration. Eliminating suture for peripheral nerve repair avoids this trauma, can decrease intervening scar tissue, and protect the repair site. Tissure's unique properties allow it to adhere to wet tissue at over five times the adhesion energy of surgical glues while also providing the flexibility to stretch through range of motion. The hydrogel membrane will resorb over the duration of nerve healing, has a demonstrated pullout strength while adhered to a nerve of over two newtons, and adheres to diverse wet surfaces including nerve tissue and collagen.
The MPWRM Resource Center was established as an interdisciplinary partnership amongst the University of Michigan, the University of Pittsburgh, and the Wyss Institute, along with technology, clinical, regulatory, marketing, and commercial translation experts from private practice and industry. Through this collaborative effort, their goal is to accelerate clinical adoption of promising technologies by providing the interdisciplinary expertise and resources to guide technologies towards FDA submissions.
"We are committed to providing clinicians innovative and effective options for the treatment of oral tissue wounds including the repair of peripheral nerves. Our initial commercialization efforts will be focused on oral surgery, but we anticipate that Tissure will fill a significant unmet need in the entire peripheral nerve market," said Robby Lane, Chief Executive Officer of Amend Surgical.
MPWRM Resource Center is one of the two national Resource Centers established by the National Institute of Dental and Craniofacial Research (NIDCR)'s Dental Oral and Craniofacial Tissue Regeneration Consortium (DOCTRC) initiative. With the overarching goal of developing clinical trial-ready tissue engineering/regenerative medicine products and protocols, the MPWRM Resource Center provides funding and resources through the Interdisciplinary Translational Project (ITP) program. The MPWRM Resource Center brings together a multi-disciplinary team of clinicians, engineers, scientists, and technology commercialization and regulatory experts from academia and industry to support the regenerative medicine research community by providing resources and expertise to guide innovations that address unmet clinical needs for the regeneration or restoration of DOC tissues. The MPWRM Resource Center is supported in part by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award Number U24DE029462.
Amend Surgical was initially funded by the Institute for Commercialization of Florida Technology which supports and funds innovation companies in Florida to solve global problems while creating high-wage jobs in new industries. Amend Surgical is focused on the development and commercialization of novel biomaterials for the oral surgical and medical device markets coupled with manufacturing expertise and operations capabilities for our OEM partnerships in the spine and orthopedics market. The company is also developing Amend Tissue Tape™ a non-resorbable hydrogel-chitosan adhesive for oral wound care which will launch in early 2024. Amend Surgical currently has nine employees and occupies a manufacturing and administrative space in the biotech corridor of Alachua, Florida.
CONTACT:
Robby Lane
Chief Executive Officer
Amend Surgical, Inc.
Related Links:
Michigan-Pittsburgh-Wyss Regenerative Medicine Resource Center
Institute for Commercialization of Florida Technology
SOURCE Amend Surgical, Inc.